| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Events |
| Progress in Electrochemical Interface for Governing Ion Permeability |
| ||||||
|
|
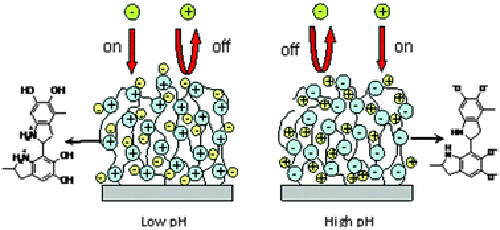
Ion permeablility of the Pdop film in solutions of different pH. (ZHOU Feng et al.)
Progress in the research on electrochemical interface for governing ion permeability has been made by the research group of material surface and interface behavior of the Lanzhou Institute of Chemical Physics (LICP) of the CAS. Pdop was spontaneously deposited on Au surfaces through self oxidative polymerization of dopamine. These membranes exhibit fully reversible, pH-switchable permselectivity for both cationic and anionic redox-active probe molecules. The pH-dependent changes in charge of the amine groups and the phenolic hydroxyl groups of the Pdop serve as independent ion gates. At high pH the film has a net negative charge that excludes anions but passes cations; at low pH it is positively charged and excludes cations but passes anions, and Pdop is responsive to kinds and concentration of electrolytes with different anions. Polyelectrolyte films exhibiting permselectivity toward electroactive counterions due to the enriched charges have long been studied. These materials are usually prone to the effects of the external environment, resulting in change in charge density and thereby altering wettability, permeability towards ions, and stiffness of films. Potential applications of such ‘‘smart’’ membranes include gates to control the permeability in microfluidic devices, surface coatings in chromatographic separations and controlled release systems, and artificial muscles to drive mechanical motion. Of the different film systems, one type with zwitterionicity that can respond to both anions and cations, particularly the pH value, would be more desirable, since they can construct a smarter interface. Pdop contains amine groups and phenolic hydroxyl groups which makes it potentially ampholytic or zwitterionic. However, this aspect has never been studied. The research was published in Chem. Commun. (Chem. Commun., 2010, 46, 5900–5902). |