New approaches for C-H and C-N bond activation have been developed by researchers from the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics, CAS. Using simpletransition-metals as catalysts, researchers have realized highly selective direct amination and alkenylation of N-containing heterocyclic compounds.
With copper as the catalyst, they have developed an efficient and conceptually new method for oxidative amination of azoles with tertiary amines. This reaction can be performed in the absence of an external base under mild conditions and only requires atmospheric oxygen as an oxidant. The catalyst system is very simple and efficient, which opens a new way for using tertiary amines as nitrogen group sources for C-N bond formation reactions. The findings have been published in Org. Lett.(Org. Lett., Vol. 13, No. 3, 2011).

Fig.1. New Strategy for Selective C-H and C-N Bond Activation (Image by HUANG Hanmin et al.)
In addition, researchers have developed the first novel iron(II)-catalyzed coupling reaction of 2-substituted azaarenes and readily accessible N-sulfonyl imines to give (E)-2-alkenylated azaarenes in high regioselectivity through cleavage of C-H and C-N bonds. This transformation provides a facile method for the synthesis of 2-alkenylated azaarenes that are of tremendous importance in medicinal chemistry. The method is simple, efficent and envorimental-friendly. The finfings have been published in Org. Lett.(Org. Lett., ASAP, DOI: 10.1021/ol200684b).
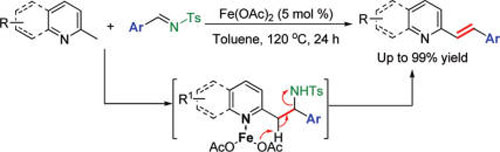
Fig.2. New Strategy for Selective C-H Alkenylation (Image by HUANG Hanmin et al.)
The above work has received support from the National Natural Science Foundation of China .
Org. Lett.(Org. Lett., Vol. 13, No. 3, 2011)Paper
Org. Lett.(Org. Lett., ASAP, DOI: 10.1021/ol200684b) Paper

