Enantioselective N-H Functionalization of Indole (Image by HUANG Hanmin et al.)
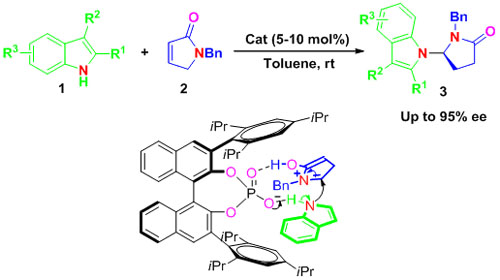
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics (LICP) have developed a novel and efficient Br?nsted acid catalyzed intermolecular enantioselective N alkylation of indoles with α,β-unsaturated γ-lactams, thus providing a highly enantioselective method for the synthesis of chiral pyrrolidinones containing indole moieties from simple starting materials. The approach opens up a new area of application of chiral Br?nsted acids toward enantioselective N-H functionalization of indoles.
Chiral indole motifs are privileged heterocyclic structures in drug discovery and widely exist in synthetic bioactive compounds and natural products. The synthesis of optically active indole derivatives through the direct enantioselective functionalization of indole cores has attracted much attention. Though many asymmetric alkylation methods exist for the functionalization of indoles at the C3 or C2 atom, the asymmetric functionalization of indoles at the N atom is limited. One way to circumvent this problem is using a base as a catalyst to facilitate cleavage the acidic proton on the nitrogen atom and make the N atom prone to alkylation. The α,β-unsaturated γ-Lactam could also act as a surrogate of the cyclic N-acyliminium ion since it could be easily converted into the N-acyliminium ion upon accepting an acidic proton from a Br?nsted acid. The conjugated Br?nsted base of chiral phosphoric acids could activate the N-H bond so the N atom acting as nucleophile attacks the N-acyliminium ion. By using this strategy the researchers have successfully installed pyrrolidinone moieties at N position of the indoles with cyclic N-acyliminium ions as electrophilies with high enantioselective.They have expatiated the mechanism of the reaction by using in situ IR, HRMS, and Deuterium-labeling experiments.
This work has received support the National Natural Science Foundation of China .
The findings have been published in Angew. Chem. Int. Ed (DOI: 10.1002/anie.201102046).
Angew. Chem. Int. Ed (Angew. Chem. Int. Ed.2011, 50, 5682 –5686) Paper
