| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Events |
| LICP Researchers Develop Series of New Catalysts and Asymmetric Catalytic Reactions |
| ||||||
|
|
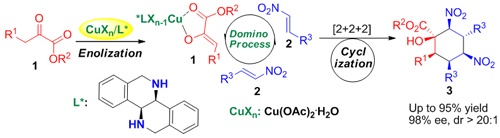
Structural motifs containing contiguous multiple chiral centers have been found in various natural products and chiral synthetic drugs. However, the number of isomers increases exponentially with the number of chiral centers, which results in the difficulty in highly stereoselective synthesis of a single isomer. And this has been one of the most challenging topics in the field of asymmetric catalysis. The research group headed by Prof. HUANG Hanmin at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, has been engaging in the research on new transitional metal catalyzed asymmetric reactions for years. They aim to develop highly stereoselective asymmetric reactions which can be used in the highly efficient synthesis of chiral molecules with biological activity via the design and synthesis of chiral catalysts and studies of their structure and performance. Through years of work, they have made a series of innovative achievements. Using the grand new chiral cyclic diamines ligands, namely biisoindoline and hexahydrodibenzo[c,h][1,5]-naphthyridine, prepared by themselves (Org. Lett.2009, 11, 4536), the group has developed for the first time copper-catalyzed highly diastereo- and enantioselective asymmetric tandem reaction for the construction of highly functionalized cyclohexane arboxylates with six stereogenic centers. The asymmetric annulation was highly enantioselective in that only one diastereomer is formed among the 64(26) stereoisomers produced with the generation of six stereocenters in this cascade asymmetric reaction, thus demonstrating the high efficiency (with diastereoselectivity>20:1 and excellent enantioselectivity (up to 95% ee)) of this protocol. The excellent performance of the present tandem reaction represents an extremely simple and efficient way to synthesize highly functionalized cyclohexanes, which may find broad applications in synthetic organic chemistry. (Angew. Chem. Int. Ed.DOI. 10.1002/anie.201107495) The research is of great significance for the deep understanding and design of new asymmetric catalytic reactions.
Asymmetric catalytic reaction for the construction of six contiguous stereocenters (Image by HUANG Hanmin et al.) Previously, using the chiral diamine ((1S,1’S)-1,1’-biisoindoline, named BIDN) also designed by themselves, they have developed a new type of efficient ruthenium catalysts containing rigid chiral diamines and achiral diphosphanes (DPPF or DPEphos) which affords excellent enantioselectivities (up to 99% ee) and reactivities (up to S/C=100 000) in asymmetric hydrogenation of simple aromatic ketones and functionalized ketones. The reaction product, chiral secondary alcohol, is a kind of important pharmaceutical intermediate. The mild reaction condition and high reactivities have provided a catalytic system with potential industrial application for the synthesis of chiral alcohol and amino alcohol compound.(Chem. Eur. J.2011, 17, 7760) Besides developing catalysts with independent intellectual property, the group also devotes itself to the development of new asymmetric catalytic reactions, including an efficient asymmetric tandem conjugate addition/Mannich reaction for the construction of multiple contiguous acyclic stereogenic centers in product with high diastereo- and enantioselectivity, an efficient asymmetric tandem dual Michael reaction that constructs three contiguous stereocenters in acyclic open-chain systems with very high enantioselectivity and diastereoselectivity, etc. This protocol provides a reliable and rapid approach for synthesis of chiral pyrrolidines with multiple stereocenters. (Angew. Chem. Int. Ed.2010, 49, 2728; Org. Lett.2011, 13, 5596) These finds may suggest an efficient strategy for controlling geometry of acyclic enolate. Synfacts, a journal engaging in providing a commented overview of the most interesting current trends in synthetic chemistry, has spoken highly of the work (Synfacts 2010, 7, 808; Synfacts 2011, 12, 1332). Moreover, the group has have also successfully established a cooperative catalytic system by the combination of an iron salt and a chiral phosphoric acid through taking advantage of the fact that the free proton source could promote the catalytic turnover for the Friedel–Crafts reaction. This newly prepared catalytic system has proven to be effective in the enantioselective Friedel-Crafts alkylation of indoles with baryl α′-hydroxy enones. (Chem. Eur. J.2010, 16, 1638) The conjugated Br?nsted base of chiral phosphoric acids could activate the N-H bond so the N atom acting as nucleophile attacks the N-acyliminium ion. By using this strategy the researchers have developed a novel and efficient Br?nsted acid catalyzed intermolecular enantioselective N alkylation of indoles with α,β-unsaturated g-lactams as electrophiles, thus providing a highly enantioselective method for the synthesis of chiral pyrrolidinones containing indole moieties from simple starting materials. The approach opens a new application route of chiral Br?nsted acids toward enantioselective N functionalization of indoles. (Angew. Chem. Int. Ed.2011, 50, 5682) Synfacts (Synfacts 2011, 8, 909) also spoke highly of the work. And the paper has become one of the 25 most accessed articles of the Angewandte Chemie in 2011. The above work has received support from the National Natural Science Foundation of China.
The paper published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2011, 50, 5682) has become one of the 25 most accessed articles from Angemandte Chemie in 2011. (Image from the Internet) |