The biology and bioinspired catalysis research group at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have synthesized novel bioinspired manganese complexes based on N4 ligands, with a more rigid, chiral diamine derived from proline and two benzimidazoles. They have successfully applied them to asymmetric epoxidation of olefins with hydrogen peroxide as a clean oxidant. Notably, 60–99?% isolated yields and excellent ee values (up to 95?%) were obtained by using low catalyst loadings (0.01–0.2 mol?%) (See Figure1). The TOF and TONs reached 59 000 h-1 and 9 600, respectively. The catalyst is one of the epoxidation catalysts with the highest activity reported up till now. 18O isotopic labeling experiments using H2O2/HOAc or peracetic acid as oxidants show incorporation of 18O into the epoxides with different degree. These observations suggest that high-valent manganese oxo species may be involved in the catalytic process.This protocol is clean, efficient, and offers a very practical method of chiral epoxide synthesis. The work has been published in Chem. Eur. J.(Chem. Eur. J.2012, 18, 6750-6753) .
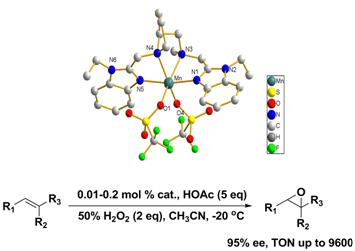
Figure 1 Asymmetric epoxidation catalyzed by new manganese complexes based on N4 ligands (Image by SUN Wei et al.)
Fe is one of the most abundant metal elements in the earth’s crust. The development of Fe catalyst is of great significance from the perspectives of sustainable and green chemistry. However, the asymmetric epoxidation of olefins promoted by Fe catalyst is currently a challenge, only few examples have been developed.
The biology and bioinspired catalysis research group has developed new non-heme iron(II) complexes of N4 ligands derived from proline and benzimidazole. The complexes exhibited an unprecedented activity and enantioselectivity for the epoxidation of a variety of di- and trisubstituted enones with up to 98% ee and 2 mol% catalyst loadings. X-ray analysis of the iron complex revealed that the ligand coordinated the iron center in cis-α topology. The 18O-isotopic-labeling experiments suggest that high-valent Fe=O species are involved as the active intermediate in the catalytic process. This system, which is based on synthetic non-heme iron catalysts, provides ready access to a wide range of epoxyketones of high enantiomeric purity. The work has been published in Chem. Eur. J.(Chem. Eur. J.2012, 18, 7332-7335) as a cover article.
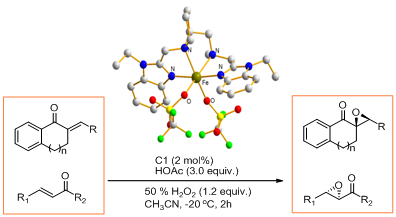
Figure 2 Asymmetric epoxidation catalyzed by new on-heme iron(II) complexes of N4 ligands(Image by SUN Wei et al.)
The above work has received support from the National Natural Science Foundation of China.

