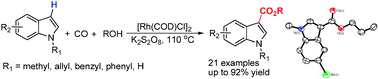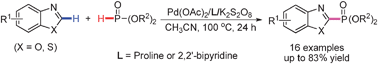| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Events |
| Researchers Accomplish Direct Carbonylation of Indoles and Direct Phosphonation of Azoles with Dialkyl Phosphites |
| ||||||
|
|
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have realized direct carbonylation of indoles and direct phosphonation of azoles with dialkyl phosphites to synthesize functionalized heterocyclic compounds which are all important organic intermediates. They have developed a Rh-catalyzed reaction protocol for the direct carbonylation of indoles to form indole-3-carboxylates. This [Rh(COD)Cl]2/K2S2O8 catalyst system showed high regioselectivity and good substance tolerance under mild conditions and without any basic or acidic additives. These features demonstrated by current methodology also point out a way forward to develop direct carbonylations of heteroarenes with other partners. The findings have been published in Chem. Comm.(Chem. Comm.2011,47,12553-12555).
Rh-catalyzed direct carbonylation of indoles to form indole-3-carboxylates (Image by LI Fuwei et al.) Then they have achieved the first palladium catalyzed oxidative phosphonation of azoles with dialkyl phosphites in high regioselectivity without additional base or acid. Such transformation represents an atom-economical alternative to traditional phosphonation of Ar–X, which requires the use of prefunctionalized arene and a stoichiometric amount of base. The reactions afford a variety of 2-phosphonated azoles in moderate to good yields and also may point a way to develop direct phosphonation of other aromatic compounds.
Palladium catalyzed oxidative phosphonation of azoles with dialkyl phosphites to form 2-phosphonated azoles(Image by LI Fuwei et al.) The above work has received support from the National Natural Science Foundation of China. The findings have been published in Chem. Comm.(Chem. Comm.2011,47, 5181-5183). |