| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Events |
| LICP Accomplishes Palladium-Catalyzed Oxidative Carbonylation of Benzylic C-H Bonds via Nondirected C(sp3)-H Activation |
| ||||||
|
|
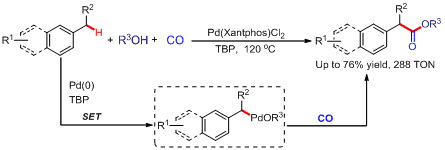
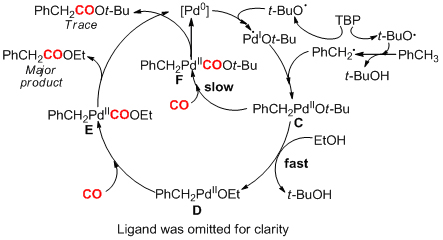
Carbon-hydrogen bond activation, especially nondirected activation of sp3C-H of simple aromatic bulk chemicals, has been a challenge in modern organic chemistry. It is of both academic significance and application potentials to develop new activation strategies of sp3C-H and apply them in the functionalization of aromatic compounds. Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have developed an efficient Pd-catalyzed carbonylation of benzylic C?H bonds with CO through nondirected C(sp3)?H bond activation. This carbonylation process represents a practical and efficient methodology for the synthesis of substituted phenylacetic acid esters from simple toluenes. Compared to the heterolysis of C-H bond, the homolysis of C-H bond requires less energy. Therefore, during the activation of inert chemical bonds, it is expected to design some homolysis processes to realize catalytic processes which cannot be accomplished through routine methods. Active free radicals generated by homolysis are a kind of important and “crazy” intermediates. It is only under special conditions that oxidative addition of low valent metal can be triggered by free radicals to form active organic reaction intermediates. In this way new catalytic reactions can be constructed. On the basis of this assumption, in this work, the benzyl radicals were firstly generated via the homolytic cleavage of the benzylic C-H under the oxidative conditions, and then benzylpalladium intermediate was formed through oxidative addition of Pd-catalyst via the single-electron-transfer (SET). The intermediate underwent CO inserted and reductive elimination to furnish the phenylacetates. Finally, phenylacetic acid was obtained after a simple hydrolysis. By using radical trapping experiments, deuterated experiment and high-resolution mass spectrometer characterization, researchers have revealed the mechanism of this new reaction as well, thus paving the way for further experiment. The work has for the first time accomplished carbonylation of sp3C-H of toluenes with high selectivity. The new strategy for generation of such a benzylpalladium intermediate should pave the way to some new classes of C?H functionalization reactions, complementary to the classical synthetic methods with organic halides. The work has received support from the National Natural Science Foundation of China . The findings have been published in J. Am. Chem. Soc.(J. Am. Chem. Soc., 2012, 134, 9902-9905) as a communication.
Scheme 1. Activation/Carbonylation of C-H (Image by HUANG Hanmin et al.)
Scheme 2. Reaction Mechanism of Activation/Carbonylation of C-H (Image by HUANG Hanming et al.) |