Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have developed a novel strategy for the Pd-catalyzed direct carbonylation of various indoles in the presence of iodine and K2CO3 under 1 atm of CO to synthesize indole-3-carboxylates in moderate to excellent yields. This methodology showed good generality to indole, alcohol, and/or phenol with high regioselectivity. Furthermore, the catalytic procedure could be conveniently applied to the systematic synthesis of biologically active tropisetron from easily available starting materials. The work has been published in Org. Lett.(2012, 14, 4130- 4133).
As far as the existing methods for direct oxidative carbonylation of C-H bonds are concerned, oxidants are often used to oxidize metal organic catalysts; while in this catalytic system, indole substrates were first oxidized by iodine to form the corresponding 3-iodoindole. In this way, the side reaction of substrates containing N-H functional group could be completely inhibited. In addition, the reduction of metal catalysts by CO and oxidation of catalytically active intermediates in catalysts could be avoided, which was a disadvantage in traditional ways of oxidative carbonylation of C-H.
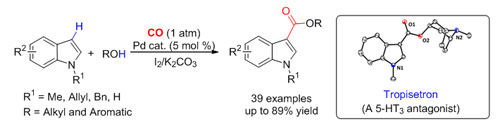
Palladium-catalyzed carbonylation to form indole-3-carboxylate (Image by LI Fuwei et al.)
Intra-molecular direct aminocarbonylation with the presence of directing groups has been reported. However, the intermolecular aminocarbonylation of the aromatic C-H bonds has rarely been reported. Using the above strategy for oxidizing substrates, the researchers have developed a novel and efficient double-carbonylation of indoles with primary or secondary amines to yield indole-3-a-ketoamides and bioactive molecules could be one-pot synthesized using the method. The system could also be selectively switched to mono-carbonylation to afford indole-3-amides only by a slight modification of reaction conditions. The work has been published in Chem. Commun.(2012, 48, 11023-11025).
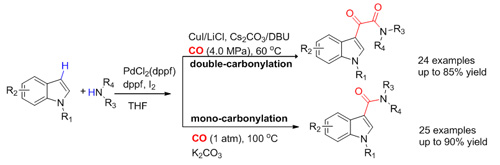
Synthesis of single/double carbonylation acetanilide-group compound catalyzed by Pd (Image by LI Fuwei et al.)
The above work has received support from the National Natural Science Foundation of China.

