| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Events |
| LICP Researchers Develop an Efficient Rh/O2 Catalytic System for Oxidative C-H Activation/Annulation |
| ||||||
|
|
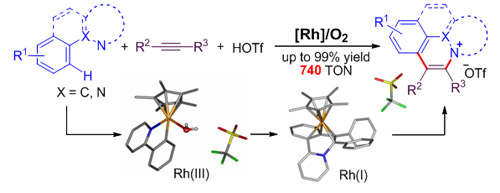
The research group headed by Professor HUANG Hanmin at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), has developed a new and efficient rhodium-catalyzed protocol for the synthesis of isoquinolinium salts from the reaction between arenes and alkynes through oxidative C−H bond activation and annulation.The work has been published in J. Am. Chem. Soc.(J. Am. Chem. Soc.2013, DOI:10.1021/ja404414q).
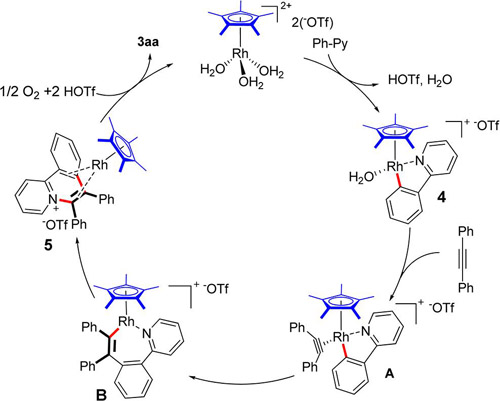
Rh-catalyzed C−H activation/aAnnulation (Image by HUANG Hanmin et al.) Usually, transition metal catalyzed oxidative functionalization of C-H is triggered by high-valent transition metal and ended by low-valent transition metal. In order to keep the reaction circulating, certain amount of oxidant has to be used to oxidize catalysts from low valence to high valence. Most commonly used oxidants are made from heavy metal, which will reduce the atom economy of the reaction and generate a large amount of heavy metal waste and eventually reduce the efficiency of the reaction. By using the Rh/O2 catalytic system and molecular oxygen as the sole oxidant, the group has not only solved the pollution caused by heavy metal but improved the activity of the reaction as well. Moreover, the group has studied the reaction mechanism and illustrated the role played by molecular oxygen by kinetic study, capture of active intermediate and X-ray crystallography.
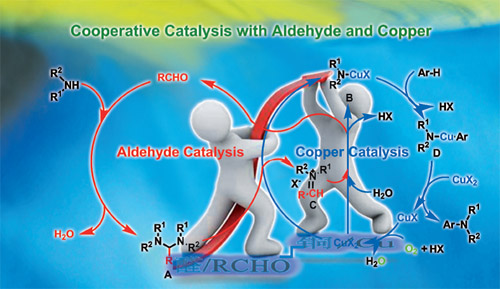
Proposed reaction pathway of Rh-catalyzed C−H activation/annulation(Image by HUANG Hanmin et al.) Hydrocarbons play an important role in modern organic chemistry. To transfer simple hydrocarbons into useful fine chemicals, medicine and organic functional materials is an ultimate goal in organic synthetic chemistry. Focusing on activation of hydrocarbons, the group has developed a series of new C-H activation reactions which have been applied in the synthesis of compounds with bioactivity. They have established a conceptually new cooperative catalytic system via a synergistic combination of aldehyde and copper catalysis. This new cooperative catalysis has been successfully applied in the direct aerobic oxidative C-H amination of azoles at room temperature, which was previously realized under harsh conditions. It not only provides an efficient method to realize the oxidative C-H amination of benzoxazoles with free amines at room temperature, but also paves the way for establishing new C-N bond formation reactions by using this efficient cooperative catalysis. The work has been published in Adv. Synth. Catal.(Adv. Synth. Catal., 2013, 355, 1315 ) as a cover paper.
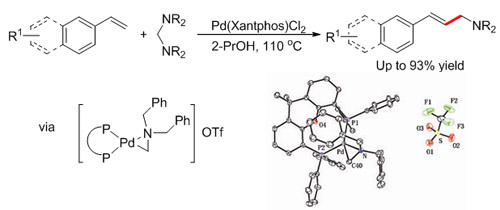
Oxidative C-H amination (Image by HUANG Hanmin et al.) In addition, the group has established a novel, highly selective palladium-catalyzed vinylation reaction for the direct synthesis of allylic amines from styrenes and aminals. The method can be directly applied in synthesizing medicines which can cure cerebral stroke, cerebral arteriosclerosis, seasick, etc. The work has been published inJ. Am. Chem. Soc. (J. Am. Chem. Soc. 2012, 134, 20613-20616) .
Palladium-catalyzed C-N activation(Image by HUANG Hanmin et al.) The above work has received support from the National Natural Science Foundation of China and “One-Three-Five” Strategic Planning of LICP. |