| News |
| ・Events |
| ・Int’l Cooperation News |
| ・Upcoming Events |
| Location: Home>News>Upcoming Events |
| Lecture: Cobalt-Catalyzed Carbonylative Polymerization and Materials Therefrom |
| ||||||
|
|
Lecture: Cobalt-Catalyzed Carbonylative Polymerization and Materials Therefrom Lecturer: Prof. Li Jia, The University of Akron, USA Time: 16:00 PM Date: June 19 Place: Academic hall, 3rd Floor, Lihua Building Introduction of Prof. Li Jia: EDUCATION B. Sc., 1991, Lanzhou University, Lanzhou, Gansu, P. R. China. Ph. D., 1996, advised by Professor Tobin J. Marks, Northwestern University, Evanston, IL 60208. PROFESSIONAL EMPLOYMENTS
1996-1998, Postdoctoral Fellow with Professor Richard A. Andersen, University of California, Lawrence Berkeley Laboratory, Berkeley, CA 94720. 1998-2005, Assistant Professor, Department of Chemistry, Lehigh University, Bethlehem, PA 18015. 2005-2007, Senior Scientist and Group Leader, Rohm and Haas Electronic Materials, Marlborough, MA 01752. 2007-2011, Assistant Professor. 2011-present, Associate Professor, Department of Polymer Science, The University of Akron, Akron, OH 44325. AWARDS
NSF CAREER Award (2002) DuPont Young Investigator Award (2001) Lindback Minority Junior Faculty Award (2000) RESEARCH SPECIALTIES
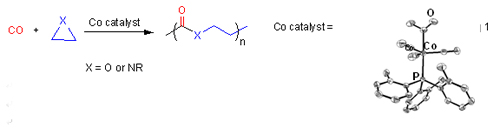
Metal-catalyzed polymerizations, supramolecular elastomers, photoresist polymers and materials, colloidal self-assembly and soft lithography. Abstract of the lecture: Cobalt-catalyzed carbonylative polymerization (COP) of heterocycles is potentially a useful method for synthesis of a wide range of polyamides and polyesters (eq 1). The presentation will first focus on the development of this class of catalytic polymerization.[i] The evolving mechanistic understanding will be discussed based on our synthetic explorations and kinetic The evolving mechanistic understanding will be discussed based on our synthetic explorations and kinetic
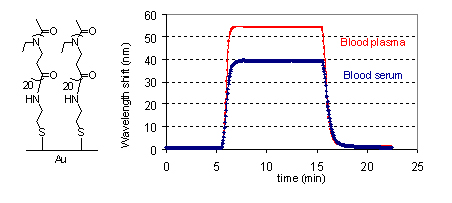
studies. The scope and limitation of the polymerization will be then discussed, including the suitable monomer types for the polymerization and the control of the microstructure and architecture of the polymer products. The properties of the poly(-alanoid)s (X = NR) synthesized via COP of aziridines will be then presented. The superb protein-adsorption resistance of the highly water-soluble members of poly(-alanoid)s (R = Me or Et) is demonstrated by surface plasma resonance spectroscopy (Figure 1).[ii]
Figure 1. Representative SPR sensorgrams showing very low level of adsorption when the poly(ethyl--alanoid)-modified surfaces are exposed to biological fluids. Finally, oligo(-alanine)s as a new physical crosslinking platform for thermoplastic elastomers will be presented. Only a few weight percent of oligo(-alanine)s is necessary for physical crosslinking to occur. The oligo(-alanine)s form filaments in the elastomeric matrix according to transmission electron microscopy. [i] J. Am. Chem. Soc. 2004, 126, 13808-13815. Ibid, 14716-14717. [ii] Biomacromolecules, 2011, 12, 2573-2582. Look forward to your participation! |