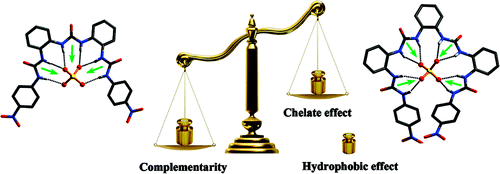
Researchers of the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics (LICP) have developed two oligourea (triurea and tetraurea) sulfate receptors by mimicking the scaffolds of the well-known tpy and qtpy ligands. Triureas possess superiority in conformational complementarity (with sulfate), whereas tetraureas benefit from both chelate effect and hydrophobic effect, resulting in stronger binding affinity in DMSO or aqueous environments, respectively.
The design of sulfate receptors is a focus in anion receptor chemistry due to their promising applications in anion templated synthesis, transmembrane anion transport, and nuclear waste remediation. Due to its environmentally and biologically related applications, selective binding of sulfate in aqueous environment attracts more attention. This is much more challenging than in organic solvents due to the severe energetic penalty paid to overcome the high hydration energy (ΔGh)-1080 kJ mol-1) of sulfate. For a long time, it was generally believed that only the receptors bearing multiple charges or charged by coordinated metal ions are able to overcome anion hydration. However, recent work has demonstrated that some neutral receptors can also possess considerable binding affinity with anions, even the greatly hydrophilic sulfate ion.
Its findings have been published in Org. Lett. (Org. Lett., Vol. 12, No. 24, 2010).
Org. Lett.Paper
