An electrophilic ring-opening reaction of 1a-type 2-alkoxy-3,4-dihydropyran with two molecules of thiophenol or thiol was developed for the first time by researchers of Lanzhou University and Lanzhou Institute of Chemical Physics (LICP) of the CAS.
The generated product contains not only a 1,3-dicarbonyl moiety but also a fragment of bis(alkylthio)methane. By using LiBr as catalyst, the dihydropyran can react selectively with thiophenol to form a ring-opening monotransthioacetalization product, which can be further converted to the final ring opening product. Therefore, a possible mechanism for the ring-opening reaction of 2-alkoxy-3,4-dihydropyran with thiophenol was proposed on the basis of postulating the ring-opening monotransthioacetalization product as an intermediate. In addition, the ring-opening monotransthioacetalization product can further react with other nucleophiles, such as indole and 2,4-dimethylthiophenol, to form the corresponding unsymmetrical double-substituted product in high yields.
Since organic sulfur compounds have become increasingly useful in organic synthesis and pharmaceutical chemistry, convenient preparations of appropriate sulfides, especially those which contain other functional groups, should be important. Although a variety of the methods for their synthesis are available in the literature, the most convenient method should involve not only transformation of a sulfur compound but also a simultaneous introduction of one or more organic functional groups into the final product. In this regard, a ring-opening reaction of epoxide with a thiol or thiophenol could be a typical example, in which both formation of C-S bond and generation of a hydroxyl group were realized at the same time. Some other ring-opening reactions with thiol or thiophenol as nucleophile showed a similar performance on the creation ofmolecular complexity,4 but unfortunately, these examples are still limited. To fit the current requirement of pharmaceutical chemistry on molecular diversity and complexity, new and efficient reactions for the synthesis of organic sulfur compounds are appealingly needed.
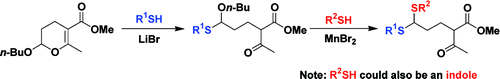
The work has received support from National Natural Science Fundation of China, Program for new Century Excellent Talents in the University of China and the Chutian Scholar Program of the Hubei provincial government. The detailed report has been published in Org. Lett. (Org. Lett., Vol. 13, No. 5, 2011).
Org. Lett. Paper
