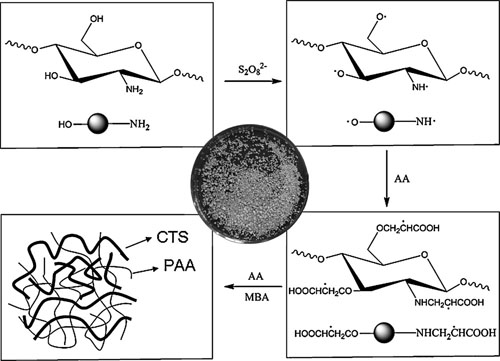
Schematic preparation of CTS-g-PAA and the digital photo of resulting product.
The recovery of a valuable metal Ni2+ was realized using CTS-g-PAA as the adsorbent by researchers from the R&D Center for Eco-material and Eco-chemistry of the Lanzhou Institute of Chemical Physics of the CAS.
The as-prepared adsorbent shows good affinity to Ni2+ ion, with the maximum adsorption capacity of 161.80mgg−1. The adsorption equilibrium can be achieved within 30 min, and the as-prepared adsorbent can be applied in a wide pH range of 3–7. This adsorbent is observed to have good adsorption capacity for Ni2+ in the presence of Cu2+ and Pb2+, and can be easily regenerated with no conspicuous losses in the adsorption capacity. Chelating interaction between the carboxylate groups and Ni2+ is the governing adsorption mechanism.
Recently, the demand for nickel in the world market is growing, while primary resources are being depleted. No matter how valuable it is, nickel is observed to produce a general toxic effect on human organism and be hazardous to the ecosystem. From the viewpoints of economical and environmental impacts, the guarantee for recovery and enrichment of nickel is rather high.
The traditional methods used in industry for recovering and separating nickel have their respective limitations: difficulty in being filtered, consuming of large amounts of organic solvent, high operation cost, low metal ions retention capacity, and long separation time. As an alternative, adsorption or solid-phase extraction is considered to be an effective method for the recovery of valuable Ni2+. Development of novel solid adsorbents for recovery of these valuable metals from aqueous solution is thus of great importance.
The work has received support from the National Natural Science Foundation of China and Science and Technology Support Project of Gansu Provincial Science and Technology Department. The findings have been published in Analytica Chimica Acta (Analytica Chimica Acta 687 (2011) 193–200).
Analytica Chimica ActaPaper
