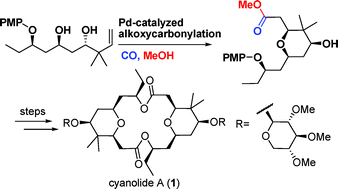
An enantioselective total synthesis of cyanolide A (1) has been achieved with the longest linear sequence of 14 steps by researchers of Lanzhou University and Lanzhou Institute of Chemical Physics (LICP) of the CAS.
Central to this venture was the efficient Pd-catalyzed intramolecular alkoxycarbonylation to construct the tetrasubstituted cistetrahydropyran ring core with high stereoselectivity. The application of SmI2-promoted Evans-Tishchenko reduction, Barbier-type reaction and Mitsunobu reaction, reduced the use of protecting groups to some certain extent. Synthesis toward cyanolide A (1) further demonstrates the value of Pd-catalyzed cyclyzation technology. It is flexible and therefore should be suitable for the synthesis of several other similar natural products.
Schistosomiasis, a disease caused by parasitic worms, is most commonly found in Asia, Africa, and South America, especially in areas where the water contains numerous freshwater snails, which may carry the parasite. Although it has a low mortality rate, schistosomiasis continues to be one of the most prevalent parasitic infections worldwide. A major problem in controlling schistosomiasis infection, however, is the complex lifecycle of the worm. The helminths require both an aquatic snail host and a mammalian host to complete their reproductive cycle. Niclosamide (Bayluscide,LC100 = 4.6µM) is the most widely used molluscicide available, effectively killing snails at all stages of the lifecycle. However, its high price, poor water solubility, and potential toxicity toward fish are considered major drawbacks. Up to now, not any molluscicides have been proved effective enough to replace niclosamide.
Cyanolide A (1), a new and highly potent molluscicidal agent against the snail vector Biomphalaria glabrata (LC50 =1.2µM), was isolated from extracts of a Papua New Guinea collection of Lyngbya bouilloni. It is a glycosidic macrolide consisting of a 16-membered macrocycle and owns a C2 symmetric structure. The interesting structure and highly potent biological activity against the snail vector Biomphalaria glabrata have attracted considerable interest in the synthetic community.
The work has received support from the Ministry of Science and Technology and National Natural Science Foundation of China. The findings have been published in Org. Biomol. Chem. (Org. Biomol. Chem., 2011, 9, 984–986).
Org. Biomol. Chem.Paper
