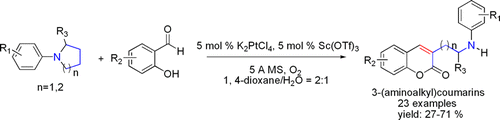
The development of novel methods for the annulation of coumarins is very important in the field of synthetic organic chemistry because coumarins are privileged structures in biological chemistry as well as important structural units found in natural and artificial products. So far, a variety of useful and efficient synthetic methods have been explored for their synthesis. However, these conventional methods are frequently restricted to relatively harsh conditions and cannot be used for substrates with sensitive groups. Thus, an effective method for the straightforward synthesis of 3-(aminoalkyl)coumarins from simple, easily available, and cheap starting materials is still in high demand in modern organic synthesis.
Researchers of Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS have a platinum/scandium cocatalyzed cascade cyclization and ring-opening reaction of tertiary amines with substituted salicylaldehydes to synthesize 3-(aminoalkyl)coumarins, which are common structural motifs found in many natural products and biologically active compounds. On the other hand, this strategy provides a useful approach to synthesize complex chiral alkamine from simple starting material. A plausible mechanism has also been proposed.
The work has received support from the National Science Foundation and the detailed report has been published in J. Org. Chem. (J. Org. Chem. Vol. 76, No. 1, 2011).
J. Org. Chem.Paper
