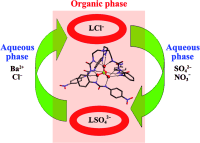
A tripodal hexaurea receptor for sulfate ions has been developed by researchers from Northwest University, South China Normal University and Lanzhou Institute of Chemical Physics of the CAS.
The receptor is capable of completely encapsulating the anion in a complementary cavity that is protected by aromatic rings, and represents a successful strategy for overcoming the “Hofmeister bias” by taking advantage of a combination of complementarity, the chelate effect], and the hydrophobic effect. With this receptor as a liquid–liquid extractant, almost quantitative extraction of sulfate ions from an aqueous to an organic phase in a recyclable manner has been achieved, and may be promising in the remediation of nuclear waste.
The design of artificial receptors for sulfate ions is of great interest because of the importance of sulfate ions in environmental and biological systems. One of the applications of sulfate ion receptors is extraction of the sulfate ion from nitrate-rich mixtures in the remediation of nuclear waste. Based on liquid–liquid anion exchange technology, extraction of sulfate ions from an aqueous to an organic phase was realized by using macrocyclic receptors. In particular, the distribution ratio (Dsulfate=[SO42-)]org/[SO42-)]aq) can reach technologically useful values (>1) when a fluorinated calixpyrrol is used. However, high concentrations (about 1000 times SO42-) of the receptor were needed in this case to ensure applicable extraction.
The detailed report has been published in Angew. Chem. Int. Ed.(Angew. Chem. Int. Ed. 2011, 50, 486 –490).
Angew. Chem. Int. Ed.Paper
