Researchers from the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics have investigated photocatalytic hydrogen evolution over Cd0.5Zn0.5S using glucose as an electron donor from NaCl saltwater, because NaCl is a major component of natural seawater.
The results show that the surface species at Cd0.5Zn0.5S exist mainly in ≡Zn-OH or ≡Zn-O- and ≡Cd-OH or ≡Cd-O- under basic condition (0.10 mol L-1 NaOH). Na+ can be adsorbed strongly at Cd0.5 Zn0.5S at high salinity. The glucose is adsorbed at Cd0.5Zn0.5S via two modes: direct adsorption at Zn and Cd sites, and indirect adsorption via adsorbed Na+ ions. With increasing NaCl concentration, the adsorption amounts of glucose and Na+ increase, which is beneficial to photocatalytic hydrogen evolution. Electrolyte NaCl can promote markedly photocatalytic hydrogen evolution, which is very important to practical application. The photocatalytic activity for hydrogen evolution from 3.0 mol L-1 NaCl saltwater increases by 77% compared to that from pure water. Photocatalyst Cd0.5Zn0.5S is stable in the saltwater reaction system.
Hydrogen is an ideal energy carrier. Photocatalytic splitting of water (PSW) using a heterogeneous photocatalyst has been studied extensively as a potential method to supply hydrogen from sunlight and water. From the viewpoint of practical application, producing hydrogen from natural seawater would be highly desirable. However, few studies on the hydrogen production from natural seawater have been made.
The work has received support from the National Basic Research Program of China, National Nature Science Foundation of China, Specialized Research Fund for the Doctoral Program of Higher Education of China and Research Fund of Education Ministry of Jiangxi, China.
The findings have been published in International Journal of Hydrogen Energy (International Journal of Hydrogen Energy 36 (2011) 4291-4297).
International Journal of HydrogenEnergy Paper
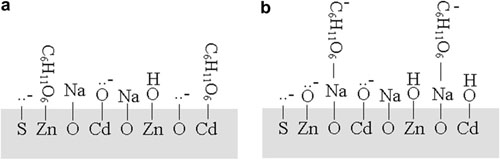
Schematic illustration of the glucose adsorption at Cd0.5Zn0.5S surface. (a) direct adsorption at the Zn and Cd sites, (b) indirect adsorption via adsorbed NaD ions.
