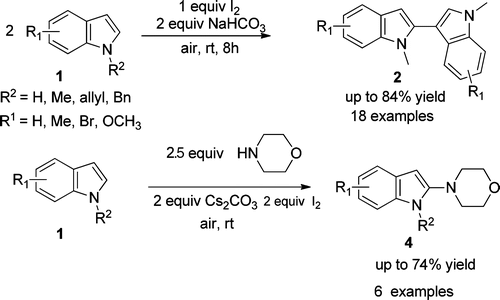
A mild, metal-free, and environmentally benign iodine-promoted regioselective C-C and C-N bonds formation of N-protected indole derivatives giving 2,3′-biindoles 2 and 4-(1H-indol-2-yl)morpholines 4 is successfully demonstrated by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS. Various bioactive 2,3′-biindoles and 4-(1Hindol-2-yl)morpholines, bearing electron-rich to moderately electron-poor substituents, can be prepared in moderate to good yields.
Since the first preparation of indole by Baeyer in 1866, its chemistry has been extensively investigated as a consequence of its prevalence in the structures of many biologically active natural products, including some useful drugs. However, the direct formation of C-C and C-N bonds of indole derivatives is particularly challenging because of the poor control of both the chemoand regioselectivities. Synthesis of 2,3′-biindolyl and 4-(1H-indol-2-yl)morpholine scaffolds, which are useful structural units that are frequently found in pharmaceuticals and functional materials, look quite attractive. Both chemical and enzymatic synthetic methods have been reported for the construction of biindolyls. Recently, a few publications have been reported by employing transition metal-catalyzed cross coupling reaction and direct functionalization of the indole derivatives to afford biindolyls. Most of these procedures require expensive metal catalysts and high loading of metal oxidants and more attention has been payed to the direct C-C bond formation of indoles. However, examples of the direct functionalization of indoles with selective C-N bond formation are rarely reported.
Therefore, a new and effective practical reaction system, involving mild, environmentally benign, atom economic, and metal-free conditions, for the regioselective construction of C-C and C-N bonds of N-protected indole derivatives is still of high demand in modern organic synthesis.
The work has been financially supported by the National Science Foundation of China. The detailed report has been published in J. Org. Chem.( J. Org. Chem. 2011, 76, 744–747).
J. Org. Chem.Paper