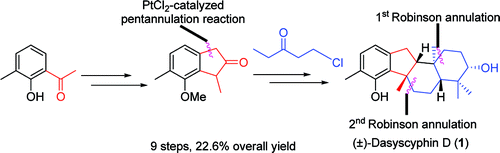
A concise and efficient total synthesis of (±)-dasyscyphin D has been accomplished in 9 steps with 22.6% overall yield by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS.
The synthesis features two points: (1) preparing the 2-indanone 3 by a PtCl2-catalyzed pentannulation reaction; (2) constructing the fused cyclic system by a double acid-catalytic Robinson annulation, which transformed 2-indanone into a tetracyclic [6-5-6-6] skeleton and installed C(8) and C(10), two all-carbon quaternary stereocenters. It is expected that the synthetic efforts will pave the way for the syntheses of other members of the dasyscyphins family.
Dasyscyphins (A-E) bear a unique [6-5-6-6] fused tetracyclic skeleton and are a family of tetracyclic terpenoid natural products possessing potent cytotoxic andmoderate antimocrobial activities (Figure 1). Among them, dasyscyphin D, isolated from Dasyscyphus niveus by Till Opatz and co-workers in 2008, inhibits the germination of conidia of Magnaporthe grisea at 20 μg/mL and imbeds five stereogenic centers, which contain two quaternary stereocenters. These intriguing structural features, along with low availability from natural sources, have attracted considerable attention to define this compound as a synthetic target.
The work has received support from the Ministry of Science and Technology of China and the National Natural Science Foundation of China. The findings have been published in Org. Lett. (Org. Lett., Vol. 13, No. 11, 2011).
Org. Lett.Paper
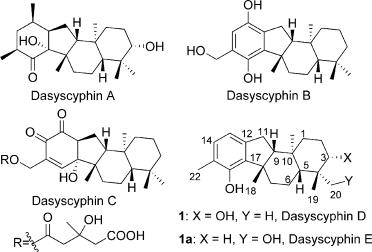
Structures of dasyscyphin A-E.

