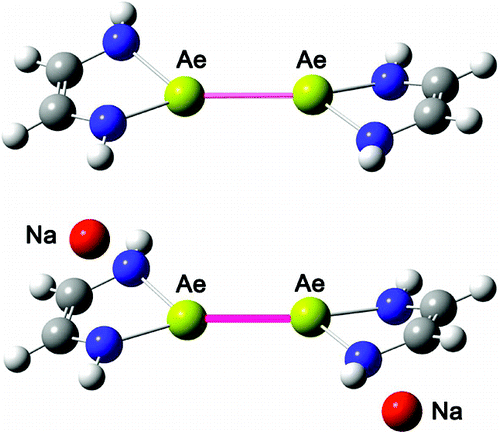
Binuclear alkaline earth metal-metal bonds (from Be to Ba) have been strongly stabilized by the anionic ligand [Na-α-diimine]- by researchers from Lanzhou Institute of Chemical Physics, South China Normal University and the University of Georgia .
The electronic structure of these five binuclear compounds (demonstrated by NBO analysis) clearly indicates covalent single bonding between metal-metal and ionic bonding between the alkali metal (Na) and the α-diimine ligand. Despite the shorter metal-metal bonds in (CHNH)2Be-Be(CHNH)2 and (CHNH)2Mg-Mg(CHNH)2, the stabilizing effects of the alkali metal on the Ae-Ae bonding is significant in view of the higher dissociation energies of the [Na]2[(CHNH)2Ae-Ae(CHNH)2] compounds compared to the (CHNH)2Ae-Ae(CHNH)2 species.
The computational study has extended the new bis-metallic complexes of Ae elements beyond the metallocenes, which not only can contribute to a deeper understanding of the Ae-Ae bonds but also may provide guidelines for future synthetic work in this field.
The work has received support from the National Natural Science Foundation of China. The findings have been published in Organometallics (Organometallics 2011, 30, 3113–3118).
OrganometallicsPaper
