The indole ring system exists ubiquitously in naturally occurring products, and many indole-containing compounds exhibit important biological and pharmaceutical activities. The synthesis and derivatization of indoles has, therefore, been a major area of focus for organic chemists over the years. As an electron rich heteroaromatic system, indole has often been used as a model substrate in electrophilic aromatic substitutions. Generally, the most reactive position of indole in these reactions is the C-3 site. Although the high reactivity can render indoles readily applicable in many reactions, when an electrophile that contains two or more reactive sites was used, how to control the reaction selectivity has become a great challenge.
Researchers from the Huazhong University of Sciences and Technology and Lanzhou Institute of Chemical Physics have proved that MnCl2 tetrahydrate is an effective catalyst for selective transformations of indoles in the presence of a keto carbonyl group. Although the side reaction between the keto carbonyl and C-3 unsubstituted indole is normally catalyzed by acid, some acid-catalyzed reactions, such as the ring opening reaction of 2-butoxy-3,4-dihydropyran with indole and transesterification of a β-keto ester with an alcohol that contains a C-3 unsubstituted indole fragment, could be performed smoothly by using MnCl2·4H2O as catalyst. A new multicomponent reaction of indole, 3,4-dihydropyran and 9a was also developed with catalysis of MnCl2·4H2O.
This work also indicated that MnCl2 might be able to play a more important role in homogeneous catalysis than we have expected before.
The work has received support from the National Natural Science Fundation of China, the Specialized Research Fund for the Doctoral Program of Higher Education, the Program for New Century Excellent Talents in the University of China and the Chutian Scholar Program of the Hubei provincial government.
The findings have been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2011, 353, 1551 – 1564).
Adv. Synth. Catal.Paper
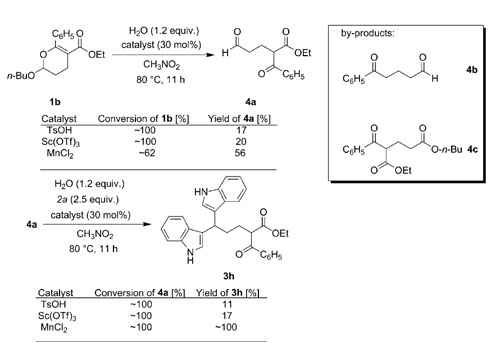
Subdivisions of the model reaction and the by-products identified in the reaction systems.
