Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics have developed two catalytic systems based on the same chiral diamine derived from l-tartaric acid for the addition of TMSCN to aldehydes by employing relatively low catalyst loading and 1.05 equiv. of TMSCN.
The performance of bifunctional catalyst 4 and catalyst 5 in combination with 3 is compared. A variety of aldehydes were converted into the corresponding trimethylsilyl ether of cyanohydrins in good yields with moderate to excellent enantioselectivities. Bifunctional catalyst 4, bearing an Noxide group in the ligand, shows better catalytic activity than complex 5 even at low catalyst loading. However, the asymmetric induction ability of catalyst 4 is inferior to 5 together with N-oxide compound 3.
These studies also indicated that an entire reversal of enantioselectivity was achieved by adding an N-oxide group onto the ligand.
Chiral cyanohydrins are versatile building blocks, because their two functional groups can be readily manipulated to produce a large range of biologically important compounds. For this reason, design and synthesis of chiral catalysts for asymmetric addition of cyanide to carbonyl compounds to form cyanohydrins is of current interest in synthetic chemistry. In recent years, metal complexes of chiral N-oxide have been disclosed as highly effective bifunctional catalysts in many asymmetric procedures.
The work has received support from the the Chinese Academy of Sciences and National Natural Science Foundation of China. The findings have been published in Eur. J. Org. Chem. (Eur. J. Org. Chem. 2011, 3407–3411).
Eur. J. Org. Chem.Paper
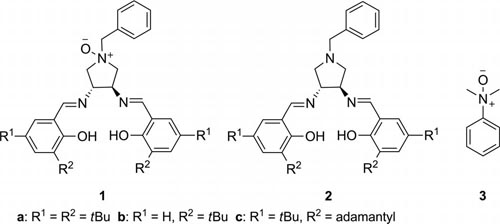
Achiral N-oxide and chiral ligands used in this study.
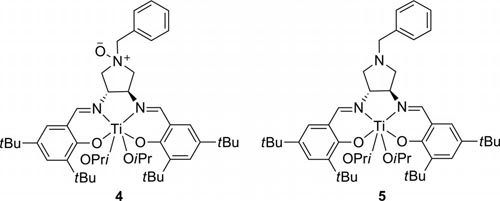
Ti complexes 4 and 5 derived from ligands 1a and 2a,respectively.

