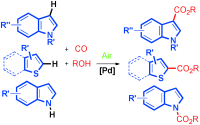
Heterocyclic carboxylic acids and derivatives are important structural motifs that are widely found in pharmaceutically important molecules and synthetically useful fine chemicals. However, direct C-H carbonylation of heteroarenes to construct an ester functional group has remained almost unexplored.
Researchers at Wuhan University and Lanzhou Institute of Chemical Physics of the CAS have developed an efficient protocol for aerobic oxidative CH carbonylation of heteroarenes to form a variety of heterocyclic esters using a balloon pressure of CO.
High regioselectivity was obtained and an electrophilic palladation mechanism was proposed. Compared with the classical carbonylation, the oxidative carbonylation involving ArH and CO/ROH in the presence of air as the oxidant presents an environmentally benign protocol for the synthesis of carboxylic compounds. In addition, this oxidative carbonylation catalytic system was also applied in the aerobic N-H carbonylation of N-free indoles to afford various indole carbamates. Further studies, with regards to substrate scope and mechanism, are currently underway and will be reported in due course.
The work has received support from the National Natural Science Foundation of China, National Program on Key Basic Research Project of China (973 Program), “the Fundamental Research Funds for the Central Universities”, and Program for New Century Excellent Talents in University (NCET).
The findings have been published in Chem. Eur. J. (Chem. Eur. J. 2011, 17, 9581 – 9585).
Chem. Eur. J.Paper