Researchers from Institute of Chemistry of the Chinese Academy of Sciences (CAS), Department of Organic Chemistry (Organisch-Chemisches Institut), University of Münster,, Germany, and Lanzhou Institute of Chemical Physics (LICP) of the CAS have found that cobalt(II) complexes bearing 8-(benzoimidazol-2-yl)quinolines, activated with methylaluminoxane (MAO), showed unique properties: ethylene oligomerization at relatively low ethylene pressure and low reaction temperature but high ethylene polymerization at 30 atm and high reaction temperature.
The resultant polymers possessed high molecular weights with narrow molecular weight distributions. The current bidentate cobalt precatalysts show good thermal stability in ethylene polymerization. The nature of the ligands finely adapted the catalytic behaviors of their cobalt complexes. Two active species could be formed for ethylene oligomerization and polymerization. Further investigations are still ongoing to determine the active species and mechanism by experimental and simulation research.
The utilization of bis(imino)pyridine complexes of iron(II) or cobalt(II) for ethylene oligomerization and polymerization has attracted much interest in both academic and industrial fields over the past dozen years. The molecular structures of cobalt(II) complexes bearing 2,8-bis(imino)quinoline ligands showed a distorted-pyramidal geometry; therefore, it is worth considering the metal precatalysts bearing bidentate ligands.
The work has received support from National High-tech R&D Program of China (863 Program). The findings have been published in Organometallics (Organometallics 2011, 30, 4847–4853).
OrganometallicsPaper
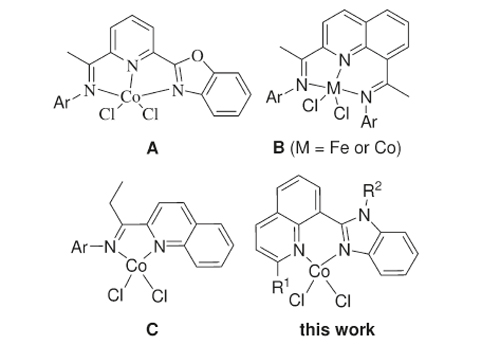
Cobalt Precatalysts for Ethylene Polymerization at Elevated Temperature
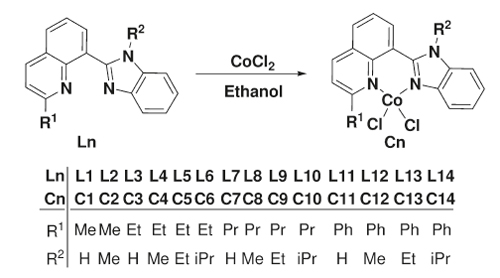
Synthesis of Cobalt Complexes

