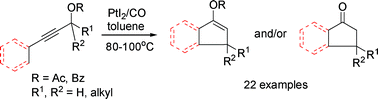
The development of new pentannulation reactions continues to be an important pursuit in synthetic organic chemistry due to the prevalence of five-membered rings in natural products. Among a variety of approaches for their preparation, the Nazarov reaction is arguably one of the most versatile and efficient methods. However, most synthetically viable applications of the Nazarov reaction have to include structural elements to control the double bond position in the enone product (C→D). In addition, strong Lewis acids and one or more equivalents of promoter are required in most cases.
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences (CAS) have developed an efficient method for constructing various 3-substituted and 3,3-disubstituted indanone derivatives via the PtI2-catalyzed tandem 3,3-rearrangement and Nazarov reaction.
This approach provides a pathway to the synthesis of natural products containing indanone skeletons. Further studies involving the synthesis of them are ongoing.
The work has received support from the Fundamental Research Funds for the Central Universities, Ministry of Science and Technology and National Natural Science Foundation of China.
The findings have been published in Org. Biomol. Chem. (Org. Biomol. Chem., 2011, 9, 7755–7762).
Org. Biomol. Chem.Paper