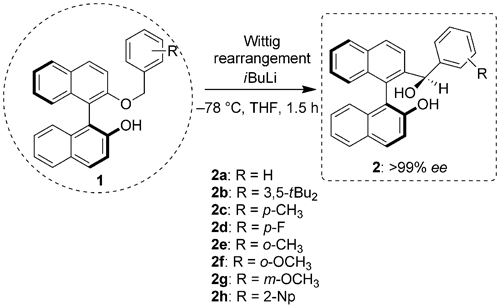
Researchers at Huangzhou Normal University and Lanzhou Institute of Chemical Physics have introduced A family of new BINOL derivatives, Ar-BINMOLs, prepared by a [1,2]-Wittig rearrangement, as new chiral molecules in the study of the mode of supramolecular aggregation on the basis of ESI-MS, NMR spectroscopy, DSC, and X-ray diffractometry.
It has been found that Ar-BINMOLs exhibit characteristics different from those of BINOL and act as supramolecules due to hydrogen bonding and aromatic π–π/C–H···π interactions. In particular, CD measurements of chiral and racemic Ar-BINMOL 2a in solution and the solid state show a strong Cotton effect, which revealed that the amplification of chirality was observed because the chiral supramolecular oligomer derived from the racemic monomer occurred.
To this end, the Ar-BINMOLs were used as supramolecular auxiliaries to mediate the Michael reaction of anthrone with (E)-β-nitrostyrene and could give the Michael adduct with comparable enantioselectivities (up to 62.5:37.5 er). In addition, the Ar-BINMOLs can act as efficient ligands to promote asymmetric 1,2-addition of diethylzinc to aldehydes, which resulted in excellent yields and enantioselectivities (up to >99.9% ee) in the reactions of diethylzinc with a broad range of aromatic aldehdyes. An NLE study showed that a monochiral center complex plays a major role in this asymmetric reaction. Further studies on the preparation and application of new ligands and catalysts based on this scaffold and basic findings are currently under way.
The preparation and application of structurally diverse, chiral compounds in asymmetric catalysis is an important goal in organic chemistry. In the field of asymmetric synthesis, 1,1′-binaphthalene-2,2′-diol (BINOL) and its derivatives, for example 2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl (BINAP), have been the most widely used compounds as a source of chirality for both stoichometric and catalytic asymmetric reactions. BINOL, one of the best known axially chiral molecules, was first shown to be a chiral auxiliary in the stoichiometric reduction of ketones with LiAlH4 in 1979, which opened the gates for axially chiral biaryls as chiral ligands, auxiliaries, and organocatalysts in asymmetric reactions. As a result, numerous C2-symmetric, axially chiral BINOL and BINAP analogs and other binaphthyl derivatives have been synthesized and tested as chiral ligands or catalysts in a variety of organic transformations. Among many successful ligands and organocatalysts, axially chiral biaryls containing both axial and sp3 central chirality are attractive.
The work has received support from the National Natural Science Foundation of China (NSFC) (20973051), the Zhejiang Provincial Natural Science Foundation of China (ZPNSFC) (Y4090139), the Program for Excellent Young Teachers in Hangzhou Normal University (HNUEYT), and the Most Important Subjects of Organic Chemistry of Zhejiang Province.
The work has been published in Eur. J. Org. Chem. (Eur. J. Org. Chem. 2011, 5039–5046).
Eur. J. Org. Chem.Paper