Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, have developed the first example of Fe complexes of N4 ligands based on the ethylenediamine backbone as catalysts for asymmetric epoxidation. These complexes display remarkably high enantioselectivities (up to 87% ee), using H2O2 or peracetic acid as oxidant, respectively.
Notably, they also indirectly demonstrated that there is a reversible water binding step prior to the generation of the most significant intermediate. Besides, [L2Fe (III)2(m-O) (m-CH3CO2)]3+ usually derived from the decay of the LFe(IV)=O species or thermodynamic sinks for a number of iron complexes was identified by HR-MS.
In addition, both path A and path B may be involved in the system and path A may be the main route to achieve the epoxidation. Further studies on expanding the scope of this catalytic asymmetric epoxidation of olefins, the detailed mechanism as well as the development of even more efficient catalysts are in progress.
Iron complexes have been extensively studied as models for non-heme iron oxygenases, demonstrating catalytic potential for the oxidation of organic substrates. Depending on the coordination sphere around the iron atom, these complexes can promote hydroxylation, epoxidation, cis-dihydroxylation, and a number of other organic transformations. Among the developed ligands, tetradentate nitrogen ligands (N4) based on an ethylenediamine backbone have been established as promising ligand frameworks.
The work has received support from the Chinese Academy of Sciences and National Natural Science Foundation of China.
The findings have been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2011, 353, 3014 – 3022).
Adv. Synth. Catal.Paper
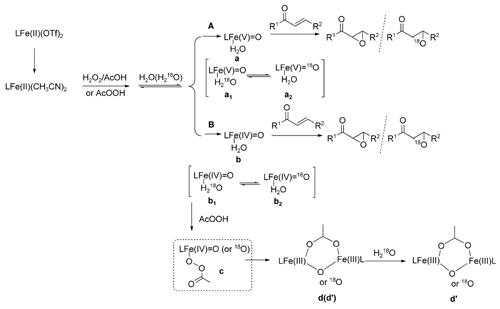
The proposed mechanism catalyzed by the designed catalytic system.
