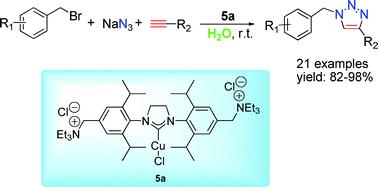
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, have developed a series of reusable ammonium salt-tagged [(SIPr)CuX] complexes, which showed highly efficient reactivity toward the three component click-reaction of a wide arrange of benzyl bromides and alkynes using water as solvent at rt. The optimized catalyst 5a could be conveniently recycled up to three times without a significant loss of its activity with catalyst loading as low as 2.0 mol% in the fresh run.
The observed higher catalytic activities of this ammonium salt-tagged [(SIPr)CuCl] compared to its standard neutral analogue demonstrates the potential of ionic functionalization of NHC– Cu(I) complex in promoting CuAAC catalysis and achieving catalyst recycle. Current experiments in their laboratory are directed at the synthesis of new recyclable transition metal-NHC complexes and their catalytic applications.
NHC-transition metal complexes have attracted considerable attention owing to their high stability and outstanding application in homogeneous catalysis. However, most of them are complicated to synthesize through multiple steps and only can be used for one time in the homogeneous reaction.
Introducing a water soluble moiety such as ammonium salt or sulfonate group onto the ligand to make the resultant metal complex more soluble in water is also an ideal solution for immobilization and recycling of the expensive (or hard to prepare) metal catalyst by using aqueous solvent system.
However, it is rather surprising that these types of NHC ligands or metal complexes have rarely been developed for catalytic reactions in water, and, to the best of researchers’ knowledge, there is no account of the synthesis of ammonium salt-tagged NHC-metal catalysts and their catalytic application using water as a green media.
Click chemistry has a wide application in chemistry, biology and material science because of its mild conditions and high efficiency. Recently, the CuAAC has been proved to be accelerated by Cu(I) species supported by nitrogen, sulfur, phosphine and NHC ligands, since these ligands could stabilize the Cu(I) center and thus enhance its catalytic activity. However, recycling of the CuAAC catalyst is scarcely investigated due to the generally homogeneous nature of these copper catalysts.
It is thus desirable to develop an efficient one-pot methodology that uses halides and sodium azide for direct cycloaddition with alkyne.
The work has received support from the National Natural Science Foundation of China and the Chinese Academy of Science.
The findings have been published in Green Chem. (Green Chem., 2011, 13, 3440–3445).
Green Chem.Paper