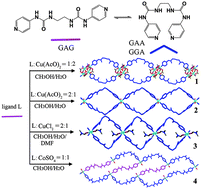
Researchers from Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences (CAS) and Northwest University have constructed four 1D coordination polymers, namely {[Cu2(AcO)3(OH)L]·2.6H2O}n (1), {[CuL2(H2O)2]·2AcO·3CH3OH}n (2), {[CuL2(DMF)2]·2Cl·2H2O}n (3), and {[Co2L3(H2O)4(CH3OH)2] ·2SO4·6H2O}n (4), from a flexible bis (pyridylurea) ligand (L).
Compounds 1, 2 and 3 are infinite 1D ribbon-like polymers with metal nodes (or metal cluster nodes in 1) and the flexible ligands in V-shaped conformations. Complex 4 shows a 1D ladder motif, in which the ligand molecules adopt two different conformations (roughly linear and V-shaped). The metal ion, counteranion, and ratio of organic ligand to metal ion show remarkable influences on the structural topologies. The urea groups and anions participate in hydrogen bonding interactions leading to the formation of higher-dimensional structures. Moreover, magnetic studies reveal that the copper(II) complex 1 has an antiferromagnetic spin–spin coupling within the tetranuclear unit.
Coordination polymers have been the subject of intense focus in the past few decades due not only to their intriguing architectures, but also because of their unique advantages in functional solid materials, ion exchange, catalysis, porous materials, magnetic and optics, etc. 1D CPs are considered to be the least structurally interesting; however, they have been found to have novel magnetic, electrical, mechanical, and optical properties. Moreover, noncovalent interactions between such 1D infinite chains can lead to interesting higher-dimensional architectures.
The work has received support from the National Natural Science Foundation of China and the findings have been published in CrystEngComm (CrystEngComm, 2011, 13, 6285–6292).
CrystEngCommPaper