Indoloquinolines have been used as antibacterial, antifungal, antimalarial, anticancer, antiplatelet, aggregation, analgesic, antihypertensive agents as well as exhibiting several other activities. Much effort has been dedicated to synthesizing polyheteroaromatic ring systems. However, most of these syntheses suffer from lack of substrate generality, use of metal catalysts, and/or involve two or more steps with exhaustive isolation/purification efforts resulting in low overall yields and hence of little interest to the pharmaceutical industry.
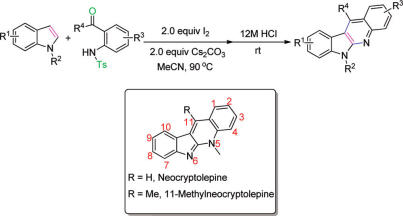
Researchers from Lanzhou University and Lanzhou Institute of Chemical Physics have a useful annulation strategy to synthesize indolo(2,3-b)quinolines from readily available indoles.
The reaction is metal-free and can produce substituted indolo(2,3-b)quinolines in a one-pot manner. The simple approach to indolo(2,3-b)quinolines complements existing routes and allows access to some novel C-11-substituted derivatives. Synthesis of neocryptolepine and its various analogues can be carried out in overall moderate yields.
The work has received support from the National Science Foundation of China, National Program on Key Basic Research Project of China (973 Program) and National "111" Project of China's Higher Education.
The findings have been published in J. Org. Chem. (J. Org. Chem. 2012, 77, 424−431).
J. Org. Chem.Paper