Oxygen is the most abundant element on the Earth and it is also one of the most important participators in the construction of both life and non-life entities. Many biological processes, which involve molecular oxygen, such as photosynthesis and metabolizations, are usually accomplished cooperatively by using a series of enzymes through complicated procedures to realize the transformation under ambient conditions.
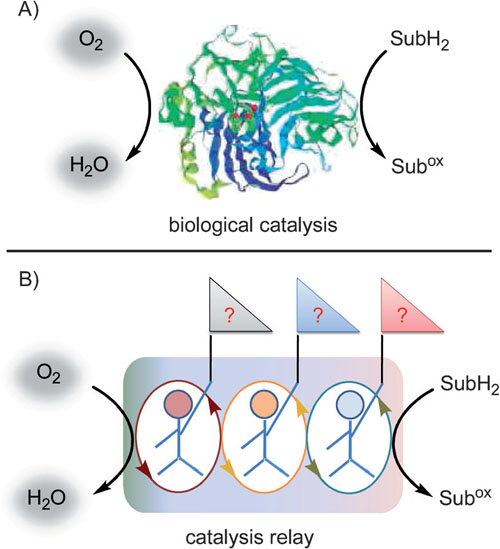 |
| Activation of molecular oxygen. A) Enzymatic oxygenase model for the transfer of an oxygen atom from molecular oxygen to the organic substrate (the picture was taken from Ref. [1]). B) Proposed catalysis relay model using small organic molecules for the transfer of oxygen atoms from O2to an organic substrate. (SubH2is the organic substrate and Subox the oxidized organic product). |
Inspired by the activation of molecular oxygen in nature, which uses multiple enzymes cooperatively, researchers from Zhejiang University of Technology, Wuhan University and Lanzhou Institute of Chemical Physics have used multiple small molecules to mimic the biological relay processes for the activation of molecular oxygen and realized the activation of molecular oxygen at ambient temperature.
Using catalytic amounts of TBN, DDQ, TEMPO, and AcOH (or TFA), O2 was successfully used as the terminal oxidant to oxidize alcohols to their corresponding carbonyl compounds. Systematic experiments indicated that all of the different catalysts cooperatively worked together similar to a relay approach to catalyze the oxidation of alcohols. Further kinetic studies revealed that the terminal oxidant O2 oxidizes NO to NO2; NO2 oxidizes DDHQ to DDQ and regenerates NO; DDQ oxidizes TEMPOH to TEMPO+ and regenerates DDHQ; and finally TEMPO+ is the substrate-selective oxidant for the oxidation of alcohols to their corresponding carbonyl products and regenerates TEMPOH. Thus, it is clear that three different catalysis processes are efficiently working together in a symbiotic catalysis relay process to achieve the activation of molecular oxygen (O2) at ambient temperatures.
The work has received support from the National Natural Science Foundations of China, National Program on Key Basic Research Project of China (973 Program), “the Fundamental Research Funds for the Central Universities”, the Program for New Century Excellent Talents in University (NCET)) and the Academic Award for Excellent Ph.D. Candidates funded by Ministry of Education of China.
The findings have been published in ChemCatChem(ChemCatChem2012, 4, 76–80).
