Ti3SiC2 is a passivated material and exhibits good corrosive resistance in common acids and alkalis. However, the tribo-corrosion behavior of Ti3SiC2 in corrosive environments has rarely been reported.
Researchers at R&D Center of Lubricating and Protecting Materials, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have investigated tribo-corrosion behaviors of Ti3SiC2/Si3N4 in hydrochloric acid (HCl) and sodium hydroxide (NaOH) with different concentrations.
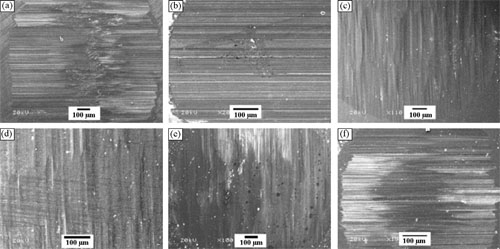 |
| SEM morphologies of worn surfaces on Si3N4in (a) 1 M, (b) 4 M, (c) 8 M HCl, (d) 1 M, (e) 10 M, and (f) 20 M NaOH solutions. |
Is has been found that in dilute and concentrated HCl and NaOH solutions, the friction coefficients of Ti3SiC2/Si3N4 vary between 0.37 and 0.17, and wear rates for Ti3SiC2 between 5X10−5 and 5X10−6mm3N−1m−1. SiO2 film forms on the worn surface of Ti3SiC2 by chemically corrosive oxidation. But the oxide film does not effectively protect the worn surface from detaching of Ti3SiC2 grains. So mechanical wear resulted from detachment of grains prevails for Ti3SiC2. Chemically corrosive reactions, in turn, decelerate mechanical friction and wear to a certain extent. Tribo-oxidation, dissolution of material, and abrasive wear are main wear mechanisms for counter Si3N4 ball. Both oxide films on Ti3SiC2 and Si3N4 are favorable for the decrease of friction coefficient. The high viscosity of NaOH solution contributes to the low friction and wear.
The work has received support from the National Natural Science Foundation of China and West Doctor Program and the Knowledge Innovation Program of the CAS.
The findings have been published in Wear(Wear274– 275 (2012) 8– 14).
