Enantiomerically pure secondary alcohols are pivotal compounds in organic synthesis, and are represented in many important target molecules, intermediates, and reagents and can be prepared by many methods. Among the many known processes, the oxidative kinetic resolution (OKR) of racemic alcohols to obtain enantioenriched alcohols is an attractive and practical method.
 |
|
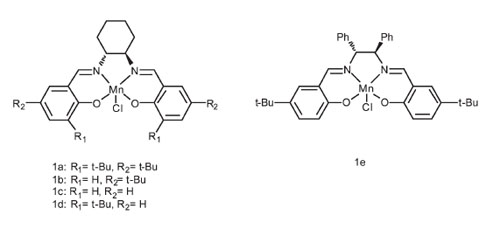
The structures of Mn(III)-salen complexes. |
Researchers at State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have demonstrate an efficient enantioselective oxidation of secondary alcohols catalyzed by Mn(III)-salen complex using N-bromosuccinimide (NBS) as the oxidant. The mild reaction conditions and enantioselectivity of the catalyst system provide access to a range of secondary alcohols including the ortho-substituted benzylic alcohols in excellent enantioselectivity. Additionally, the utilization of cheap, easily available NBS as oxidant makes the protocol more practical toward the synthesis of enantiomerically pure secondary alcohols.
The work was funded by the Chinese Academy of Sciences and the National Natural Science Foundation of China. The findings have been published in Org. Biomol. Chem.(Org. Biomol. Chem., 2012, 10, 2730–2732) .
