Abstract:
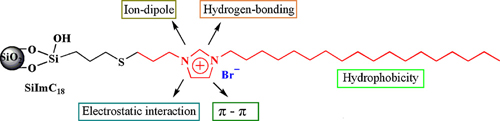
In this paper, a new imidazolium-embedded C18 stationary phase (SiImC18) for reversed-phase highperformance liquid chromatography is described. 1-Allyl-3-octadecylimidazolium bromide ionic liquid compound having a long alkyl chain and reactive groups was newly prepared and grafted onto 3-mercaptopropyltrimethoxysilane-modified silica via a surface-initiated radical-chain transfer addition reaction. The SiImC18 obtained was characterized by elemental analysis, infrared spectroscopy, thermogravimetric analysis, diffuse reflectance infrared Fourier transform, and solid-state 13C and 29Si cross-polarization/magic angle spinning nuclear magnetic resonance spectroscopy. The selectivity toward polycyclic aromatic hydrocarbons relative to that toward alkylbenzenes exhibited by SiImC18 was higher than the corresponding selectivity exhibited by a conventional octadecyl silica (ODS) column, which could be explained by electrostatic π-π interaction cationic imidazolium and electron-rich aromatic rings. On the other hand, SiImC18 also showed high selectivity for polar compounds, which was based on the multiple interaction and retention mechanisms of this phase with different analytes. 1,6- Dinitropyrene and 1,8-dinitropyrene, which form a positional isomer pair of dipolar compounds, were separated successfully with the SiImC18 phase. Seven nucleosides and bases (i.e. cytidine, uracil, uridine, thymine, guanosine, xanthosine, and adenosine) were separated using only water as the mobile phase within 8 min, which is difficult to achieve when using conventional hydrophobic columns such as ODS. The combination of electrostatic and hydrophobic interactions is important for the effective separation of such basic compounds without the use of any organic additive as the eluent in the octadecylimidazolium column.
 |
Conclusions:
A new C18 stationary phase with an embedded imidazolium group was prepared via a surface radical chain-transfer addition reaction of 1-allyl-3-octadecylimidazolium bromide with 3-mercaptopropyl silica and characterized by elemental analysis, DRIFT, and solid 13C and 29Si NMR spectroscopy. The chromatographic performance of the octadecylimidazolium silica phase and a conventional monomeric octadecyl silica phase was compared by separating alkylbenzenes and PAHs. The new phase also effectively separated dipolar compounds, bases, and nucleosides. The embedded imidazolium group is able to interact with different analytes via multiple interactions, including hydrophobic, π-π , electrostatic, hydrogen-bonding, and ion–dipole interactions. We believe that more applications of this novel type of embedded imidazolium phases will be reported.
Published in Analytica Chimica Acta738 (2012) 95– 101
