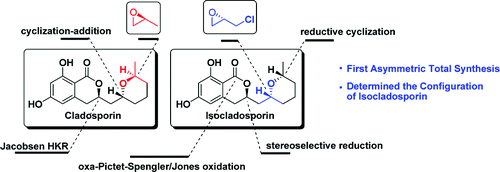
ABSTRACT: The first asymmetric total syntheses of cladosporin and isocladosporin were accomplished in 8 steps with 8% overall yield and 10 steps with 26% overall yield, respectively. The relative configuration of isocladosporin was determined via this total synthesis.
CONCLUSION
In conclusion, we have accomplished the first asymmetric total synthesis of cladosporin ((−)-1) and isocladosporin ((−)-2) from commercially available (S)-propylene oxide and (S)-epichlorohydrin as chiral sources, respectively. The strategy was developed on the basis of a notion of concise enantioselective assembly of the tetrahydropyran ring. The relative configuration of isocladosporin was defined as the structure of 2 compared with 3 through this total synthesis.
Published in J. Org. Chem. 2012, 77, 5656−5663