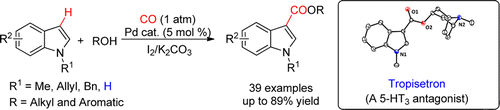
Abstract: A novel strategy involving a first oxidative iodination and subsequent Pd0-catalyzed carbonylation to yield indole-3-carboxylate has been developed. It showed perfect generality to indole, alcohol, and phenol. The current methodology could also be conveniently applied to the synthesis of biologically active tropisetron from simple indole and tropine.
Conclusion: In conclusion, a novel strategy for the Pd-catalyzed direct carbonylation of various indoles to synthesize indole-3-carboxylates in moderate to excellent yields has been successfully developed. This methodology showed good generality to indole, alcohol, and/or phenol with high regioselectivity. Furthermore, the current catalytic procedure could be conveniently applied to the systematic synthesis of biologically active tropisetron from easily available starting materials. Further explorations on the mechanism and more synthetic applications of the present method are under investigation in our laboratory.
Published in Org. Lett., Vol. 14, No. 16, 2012