Abstract: A series of ionic liquid modified hypercrosslinked polystyrene resins are synthesized by adding different quantities of imidazole in the Friedel-Crafts reaction. The resins are characterized by nitrogen adsorption/desorption, Fourier transform infrared spectroscopy, elemental analysis and scanning electron microscopy. The adsorption properties of (-)-epigallocatechin gallate and (-)-epicatechin are investigated and compared with macroporous adsorption resins Seplite D101 and Seplite AB-8. The (-)-epigallocatechin gallate and (-)-epicatechin uptakes on HP-IL16 are remarkably larger than those of macroporous adsorption resins Seplite D101 and Seplite AB-8. The maximum adsorption capacity of HP-IL16 are up to 73.1 mg g(-1) for (-)-epigallocatechin gallate and 93.0 mg g(-1) for (-)-epicatechin. The adsorption isotherms are best described by the Langmuir model, and their adsorption kinetics follow the pseudo-second-order kinetic equation and intra-particle diffusion model. Analysis of the adsorption mechanism suggest that the synergistic effect of the specific surface area, molecular sieving effect, and multiple adsorption interactions are the driving forces of adsorption. The resin is reusable and presents a good desorption rate. Based on these results, this study opens up the possibility of synthesizing ionic liquid modified hypercrosslinked polymeric resins for purification of catechin from herbal plants. Key words: AQUEOUS-SOLUTIONS; POLYMER NETWORKS; SAMPLE PREPARATION; STATIONARY PHASES; METHYL-ORANGE;CHROMATOGRAPHY; REMOVAL; PURIFICATION; PERFORMANCE; AMINO 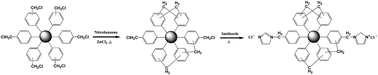
Published in RSC ADVANCES, 5 (89):72601-72609; 10.1039/c5ra08273k 2015 |
