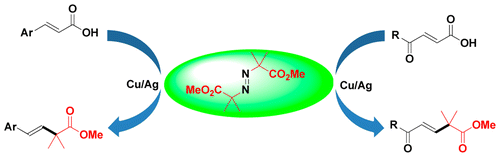
Abstract: A facile and general method for coppercatalyzed decarboxylative alkylcarboxylation of cinnamic acids with dimethyl 2,2'-azobis(2-methylpropionate) has been developed. The scope and versatility of the reaction was demonstrated, and a broad range of substrates bearing electron-donating and -withdrawing groups on the aromatic rings were all compatible with this reaction to provide desired beta,gamma-unsaturated esters in moderate to good yields. Moreover, alpha,beta-unsaturated acids with a carbonyl group on the gamma-position of acrylic acids also smoothly proceeded to furnish the desired products in good yields. KeyWords Plus: MAGNESIUM CARBENOID 1,2-CH; CARBOXYLIC-ACIDS; CINNAMIC-ACIDS; ALLYLIC ESTERS; BETA,GAMMA-UNSATURATED ESTERS; CARBONYLATION REACTIONS; CARBON-MONOXIDE; KEY REACTION; ALKENES; DERIVATIVES Published in ORGANIC LETTERS, 17 (20):4968-4971; 10.1021/acs.orglett.5b02382 OCT 16 2015
|