Abstract: A mild and convenient synthesis of carbazoles by TfOTMS (trimethylsilyl trifluoromethanesulfonate)-catalyzed ring-opening annulation of 2-amidodihydrofurans is presented with a high degree of chemoselectivity and regioselectivity. This procedure was also scaled up to a gram-scale synthesis. The reaction could involve an iminonium intermediate through a series of CO, CN bond cleavages, CC bond formations, and a 1,2-migration process. Key words: acid-catalysis; carbazoles; chemoselectivity; regioselectivity; ring-opening annulation 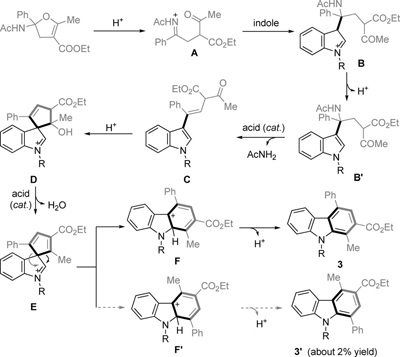
Published in CHEMISTRY-A EUROPEAN JOURNAL, 21 (46):16383-16386; 10.1002/chem.201503260 NOV 9 2015
|
