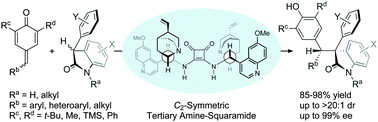
Abstract: The asymmetric catalytic 1,6-addition of p-QMs with racemic oxindoles under the bifunctional catalysis of C-2-symmetric dimeric Cinchona-derived squaramide is described. This tertiary amine-squaramide catalyzed reaction provides a diastereoselective and enantioselective approach to the effective assembly of diverse diarylmethine-substituted oxindoles having vicinal tertiary and quaternary stereocenters. KeyWords Plus: DYNAMIC KINETIC RESOLUTION; CINCHONA ALKALOID ORGANOCATALYSTS; SELF-ASSOCIATION-FREE; ENANTIOSELECTIVE SYNTHESIS; RACEMIC AZLACTONES; ALPHA-ALKYLATION; EXPEDIENT ACCESS; ALDEHYDES; DESIGN Published in CHEMICAL COMMUNICATIONS, 52 (22):4183-4186; 10.1039/c5cc10502a 2016
|