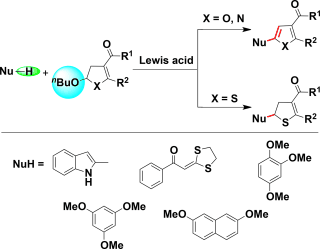
Abstract: An effective method to synthesize alpha-functionalized furan and pyrrole derivatives was developed using 2-alkoxy-2,3-dihydrofurans as modular precursors. This protocol featured a previously unreported tandem nucleophilic substitution/heteroaromatization reaction. Nucleophiles such as indole, alpha-oxoketene dithioacetal, trimethoxybenzene, and dimethoxynaphthalene can react readily with 2-alkoxy-2,3-dihydrofurans to afford a-functionalized five-membered ring heterocycles in the presence of acid catalysts, such as copper bromide and iron chloride. The mechanism of the reaction was also discussed, in which the first step, nucleophilic substitution, is the key in triggering the succeeding heteroaromatization. This method can also be extended to the synthesis of dihydrothiophenes. KeyWords Plus: ONE-POT SYNTHESIS; CATALYZED DIRECT ARYLATION; C BOND FORMATION; OXOKETENE DITHIOACETALS; PROPARGYLIC ALCOHOLS; ALPHA-ALKYLATION; INDOLE ALKALOIDS; NATURAL-PRODUCTS; HIGHLY EFFICIENT; 2-STEP SYNTHESIS Published in ADVANCED SYNTHESIS & CATALYSIS, 358 (6):900-918; 10.1002/adsc.201500993 MAR 17 2016
|