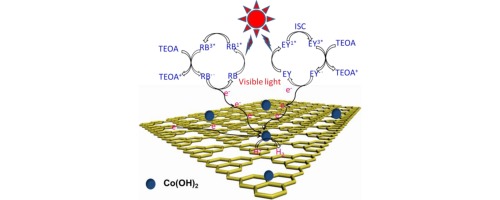
Abstract: In this work, non-noble metal cobalt hydroxide nanoparticles [Co(OH)(2) NPs] implanted uniformly on graphitic carbon nitride (g-C3N4) as a co-catalyst for hydrogen evolution reaction (HER) by in-situ chemical deposition method were reported. After co-sensitized by Eosin Y (EY) and Rose Bengal (RB) dyes, this photocatalyst exhibited a comparatively low onset potential (0.26V) for HER and high photocatalytic HER activity under visible light irradiation. The g-C3N4 not only provided a large area and nanoporous structure for the confined growth of Co(OH)(2) NPs, but also greatly facilitated EY and RB (ER) molecules assembling on its surface, which promoted the charge transfer from dye to co-catalyst. The PL spectra and lifetime test showed the strong interaction between Co(OH)(2) NPs and g-C3N4 could enhance the transferr rate of the photogenerated electron to reduce the carries recombination. About 431.9 mu,mol of hydrogen generated over ER co-sensitized Co(OH)(2)/C3N4 in 3 h. The apparent quantum efficiencies (AQE) of 29.6 and 27.3% were achieved at 520 nm and 550 nm over Co(OH)(2)/C3N4, respectively. In addition, this co-sensitized photocatalyst showed satisfied stability for HER, no remarkable decay of activity was observed in 900min reaction. These results imply that Co(OH)(2) is a stable and efficient cheaper co-catalyst for photo-HER. KeyWords Plus: GRAPHITIC CARBON NITRIDE; PHOTOCATALYTIC WATER OXIDATION; REDUCED GRAPHENE OXIDE; METAL-FREE; H-2 EVOLUTION; ELECTRON-TRANSFER; DOPED G-C3N4; CATALYSTS; COCATALYST; COBALT Published in APPLIED CATALYSIS B-ENVIRONMENTAL, 188 56-64; 10.1016/j.apcatb.2016.01.057 JUL 5 2016
|