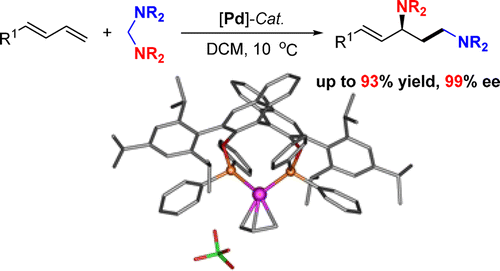
Abstract:A novel highly enantioselective amino-methylamination of conjugated dienes with aminals catalyzed by a chiral palladium complex ligated with BINOL-derived chiral diphosphinite has been successfully developed. This reaction proceeds via a Pd-catalyzed cascade C-N bond activation, aminomethylation, and asymmetric allylic amination reaction under mild reaction conditions, providing a unique and efficient strategy for the synthesis of enantiomerically pure allylic 1,3-diamines. KeyWords Plus:DIRECT MICHAEL ADDITION; BETA-KETO-ESTERS; ASYMMETRIC-SYNTHESIS; STEREOSELECTIVE-SYNTHESIS; TERMINAL 1,3-DIENES; HECK REACTION; ARYL HALIDES; ALKENES; NITROALKANES; DIBORATION Published in JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 138 (13):4314-4317; 10.1021/jacs.6b00976 APR 6 2016
|