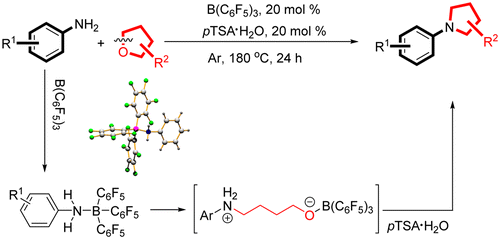
Abstract:A metal-free and efficient approach to N-aryl substituted azacycles from arylamines and cyclic ethers is described. In this synthesis, the synergistic effect between Lewis and Bronsted acids is crucial to the ring-opening of cyclic ethers and the subsequent cydization. The use of B(C6F5)(3) enabled the formation of frustrated Lewis pairs (FLPs) from the reactants, and the resulting FLPs allowed ready access to the N-arylazacycles in moderate to good yields via further cydization. Water is the sole waste resulting from the reaction, thereby making it an environmentally benign process. KeyWords Plus:FRUSTRATED LEWIS PAIRS; HIGHLY ENANTIOSELECTIVE HYDROGENATION; C-H BOND; PRIMARY AMINES; EFFICIENT; REACTIVITY; BORANE; ALKYLATION; AMINATION; ARYLATION Published in ORGANIC LETTERS, 18 (7):1522-1525; 10.1021/acs.orglett.6b00157 APR 1 2016
|