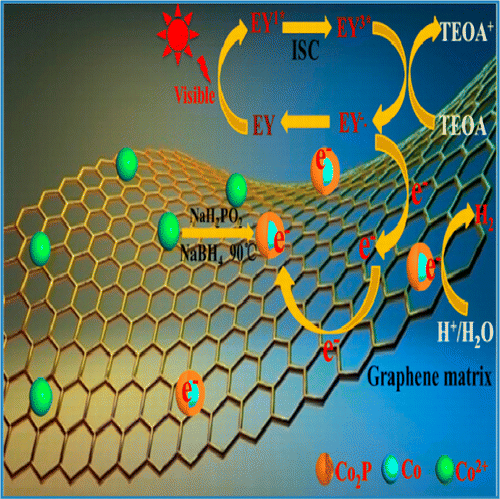
Abstract: We show that photocatalytic hydrogen evolution reaction (HER) rate is highly dependent on Co surface state, indicated by binding energy data. The key process of hydrogen generation, Co2P-H species formation, follows lower hydrogen adsorption free energy (Delta G(H)) route. Such low surface energy species can dramatically decrease the overpotential for HER (about 35 mV for HER in basic electrolyte at pH 11, and 150 and 196 mV overpotentials at current density 5 and 15 mA/cm(2), respectively). This could explain why Co2P loaded on reduced graphene oxide (RGO) reached high hydrogen generation rate, 1068 mu mol.h(-1), much higher than that of Pt/RGO catalyst (822 mu mol.h(-1)) under the same reaction condition, while a high apparent quantum efficiency (AQE) (33.3%) was achieved at 520 nm. Moreover, it opens a design strategy for development of cocatalyst with enhanced efficiencies through change of surface H species formation. KeyWords Plus: CATALYST; WATER; IRRADIATION; CHALLENGES; REDUCTION; PHASE Published in JOURNAL OF PHYSICAL CHEMISTRY C, 120 (12):6409-6415; 10.1021/acs.jpcc.6b00680 MAR 31 2016
|