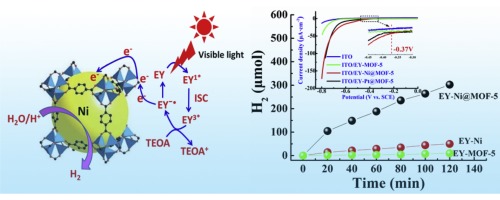
Abstract: The development of an advanced co-catalyst is critical for improving the efficiency of the photocatalytic hydrogen evolution reaction. Noble metals (such as Pt) have been identified to be the most active co-catalyst for this reaction since they exhibit significantly low over-potential. However, their low-abundance, high cost, its scale-up setup usage is impeditive. Here, we reported that high efficient small-sized nickel particles embedded in the frameworks of MOF-5 as a co-catalyst with low over potential for visible photocatalytic hydrogen evolution. The electrochemical measurements showed a low over-potential of -0.37 V, which have the similar over-potential as Pt@MOF-5. Such low over-potential is attributed to their high dispersion (41.8%), small-sized nickel particles (similar to 9 nm), and high specific surface area of MOF-5 (2973 m(2)/g). As evidenced by electrochemical impedance spectra (EIS) measurements, Ni nanoparticles with exposed (11 1) facet were more benefited the electrons transfer from MOF-5 than that of (2 0 0) facet one. In addition, Ni@MOF-5 exhibited the larger transient photocurrent and longer fluorescence lifetime. These results led to the high photocatalytic activities and stability for hydrogen evolution sensitized by Eosin Y (EY) over Ni@MOF-5. The rate of hydrogen evolution reached 30.22 mmol h(-1) g(-1)[Ni] (at pH 11) over Ni@MOF-5 irradiated under visible light irradiation (lambda >= 420 nm) in 2 h. The apparent quantum efficiency (AQE) of 16.7% over EY-Ni@MOF-5 was achieved at 430 nm. MOF-5 might promote the photogenerated electrons transfer from excited EY to the hydrogen evolution active sites (Ni), and consequently enhance photocatalytic hydrogen evolution efficiency. Keywords: Small-sized Ni(111) particles; Metal-organic frameworks; Low over-potential; Visible photocatalytic hydrogen generation Published in APPLIED CATALYSIS B-ENVIRONMENTAL, 190 12-25; 10.1016/j.apcatb.2016.02.061 AUG 5 2016
|