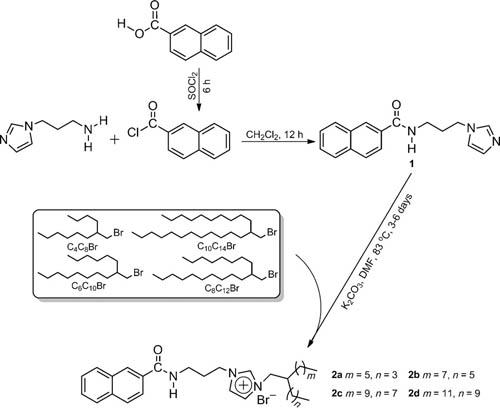
Abstract: Obtaining pi-conjugated room temperature ionic liquids (RTILs) is difficult because of the relatively strong pi-pi interaction among the pi-moieties. Existing strategies by using bulky counterions greatly hindered further property optimization and potential applications of these intriguing functional fluids through simple ion exchange. Herein, four naphthalene-functionalized, pi-conjugated RTILs with small counterions (Br-) have been facilely synthesized with high yields. Our strategy is to attach branched alkyl chains to the cationic backbone of the target compounds (2a-d), which effectively tune inter-and intramolecular interactions. Compounds 2a-d have satisfactory thermal stability (up to 300 degrees C) and low melting points (< -198 degrees C). Rheological measurements revealed the fluid character of 2a-d, whose viscosity decrease with the increase of the alkyl chain length and temperature. The presence of the pi-conjugated naphthalene moiety imparts 2a-d photoluminescent properties in bulk solutions. Moreover, the absence of strong pi-pi stacking among the naphthalene units in solvent-free states enables them to be used as a new generation of photoluminescent inks. Keywords: branched alkyl chains; imidazole; ionic liquids; naphthalene; photoluminescence Published in CHEMISTRY-A EUROPEAN JOURNAL, 22 (18):6286-6293; 10.1002/chem.201504764 APR 25 2016
|