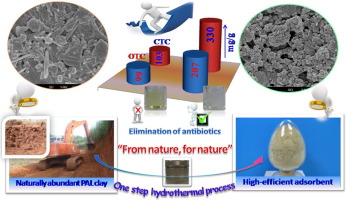
Abstract: As promising eco-friendly materials, natural silicates have received great attention as abundant, low-cost, non-toxic, stable, and environmentally benign adsorbents. Inspired by the idea of "from nature, for nature", a series of highly efficient hybrid silicate adsorbents were synthesized via a simple one-step hydrothermal process, using naturally abundant low-grade palygorskite (PAL) as the initial material in the presence of sodium silicate (SS), magnesium sulfate (MS), and monochloroacetic acid (MCA). As expected, the PAL crystal and the associated minerals were restructured as amorphous and multi porous Mg, Al-silicates, while the active -COOH groups were simultaneously introduced into the silicate to form a hybrid adsorbent with a specific surface area of 410.61 m(2)/g (compared to 52.87 m(2)/g for raw PAL). The hybrid silicate adsorbent showed excellent adsorption capabilities for the antibiotics chlortetracycline (CTC) (329.84 mg/g) and oxytetracycline (OTC) (207.47 mg/g), which were enhanced by 218.9% and 107.9%, respectively, in contrast to that of raw PAL. The adsorption of the hybrid adsorbent for CTC and OTC was pH-dependent, and the pH values of 3.56-7.82 (for CTC) and 3.45-7.57 (for OTC) favored the adsorption. The dynamic adsorption process was well described by a pseudo second-order model, which suggested that chemical adsorption was the prominent driving force. The thermodynamic adsorption pattern agreed well with the Redlich-Peterson model, revealing that the heterogeneous surface was the main adsorption site and that removal of antibiotics occurred mainly by multi-layer adsorption. KeyWords Plus:STEP HYDROTHERMAL PROCESS; METHYLENE-BLUE; AQUEOUS-SOLUTION; TETRACYCLINE ANTIBIOTICS; ACTIVATED CARBON; MONTMORILLONITE CLAY; SURFACE-AREA; ADSORPTION; KINETICS; SEPIOLITE Published in CHEMICAL ENGINEERING JOURNAL, 293 376-385; 10.1016/j.cej.2016.02.035 JUN 1 2016
|