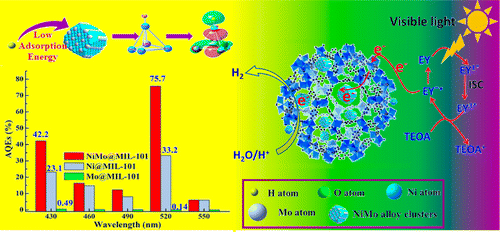
Abstract: An effective cocatalyst is crucial for enhancing the visible photocatalytic performance of the hydrogen generation reaction. By using density-functional theory (DFT) and frontier molecular orbital (FMO) theory calculation analysis, the hydrogen adsorption free energy (Delta G(H)) of Ni-Mo alloy (458 kJ.mol(-1)) is found to be lower than that of Ni itself (537 kJ.mol(-1)). Inspired by these results, the novel, highly efficient cocatalyst NiMo@MIL-101 for photocatalysis of the hydrogen evolution reaction (HER) was fabricated using the double solvents method (DSM). In contrast with Ni@MIL-101 and Mo@MIL-101, NiMo@MIL-101 exhibited an excellent photocatalytic performance (740.2 mu mol.h(-1) for HER), stability, and high apparent quantum efficiency (75.7%) under 520 nm illumination at pH 7. The NiMo@MIL-101 catalyst also showed a higher transient photocurrent, lower overpotential (-0.51 V), and longer fluorescence lifetime (1.57 ns). The results uncover the dependence of the photocatalytic activity of HER on the Delta G(H) of Ni-Mo (MoNi4) alloy nanoclusters, i.e., lower Delta G(H) corresponding to higher HER activity for the first time. The NiMo@MIL-101 catalyst could be a promising candidate to replace precious-metal catalysts of the HER. KeyWords Plus: ELECTRONIC-STRUCTURE; CATALYTIC-ACTIVITY; LIGHT IRRADIATION; ALKALINE-SOLUTION; CARBON NANOTUBES; CHARGE-TRANSFER; NICKEL; WATER; GRAPHENE; ALLOYS Published in ACS APPLIED MATERIALS & INTERFACES, 8 (17):10808-10819; 10.1021/acsami.5b12524 MAY 4 2016
|