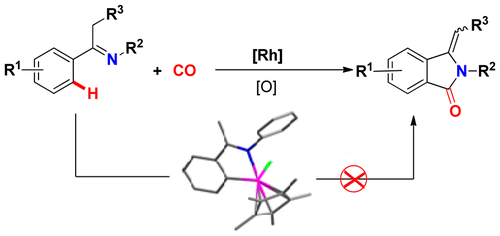
Abstract: A novel rhodium-catalyzed oxidative cyclocarbonylation of ketimines via cleavage of two C-H bonds was established, which provided a direct and reliable method for the synthesis of a wide range of 3-methyleneisoindolin-1-ones with mostly moderate yields. Preliminary experimental mechanistic studies and DFT calculations revealed that this reaction proceeds via imine-enamine tautomerization, N-H cleavage, C-H bond activation, CO insertion, and reductive elimination. The mechanism studies further ruled out an isolated cyclometalated rhodium complex being involved in the present reaction, which was different from many other documented rhodium-catalyzed C-H cyclization reactions. KeyWords Plus: ONE-POT SYNTHESIS; OXIDATIVE CARBONYLATION; C(SP(3))-H BONDS; REGIOSELECTIVE CARBONYLATION; STEREOSELECTIVE-SYNTHESIS; EFFICIENT SYNTHESIS; 3+2 CYCLOADDITION; ALIPHATIC-AMINES; MOLECULAR-OXYGEN; TERMINAL ALKYNES Published in ORGANOMETALLICS, 35 (10):1480-1487; 10.1021/acs.organomet.6b00072 MAY 23 2016
|